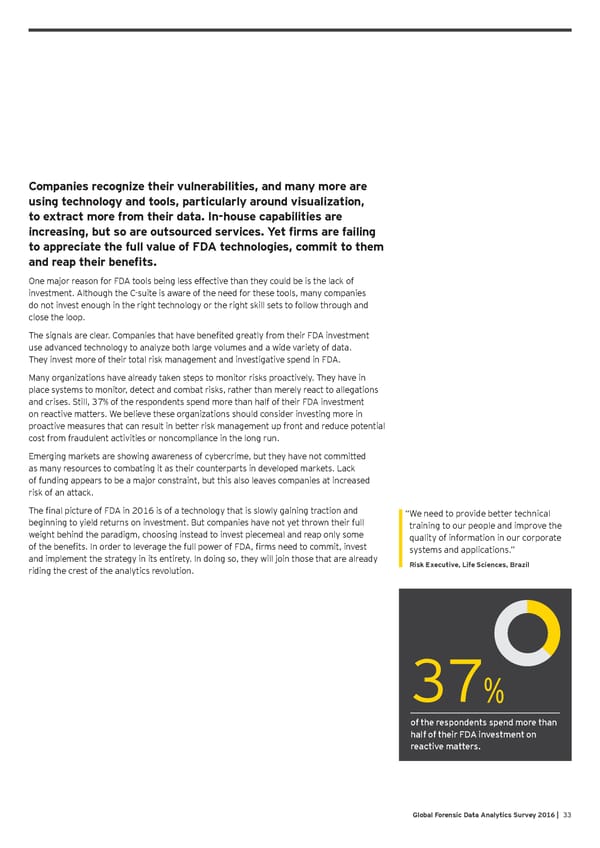
Companies recognize their vulnerabilities, and many more are using technology and tools, particularly around visualization, to extract more from their data. In-house capabilities are increasing, but so are outsourced services. Yet firms are failing to appreciate the full value of FDA technologies, commit to them and reap their benefits. One major reason for FDA tools being less effective than they could be is the lack of investment. Although the C-suite is aware of the need for these tools, many companies do not invest enough in the right technology or the right skill sets to follow through and close the loop. The signals are clear. Companies that have benefited greatly from their FDA investment use advanced technology to analyze both large volumes and a wide variety of data. They invest more of their total risk management and investigative spend in FDA. Many organizations have already taken steps to monitor risks proactively. They have in place systems to monitor, detect and combat risks, rather than merely react to allegations and crises. Still, 37% of the respondents spend more than half of their FDA investment on reactive matters. We believe these organizations should consider investing more in proactive measures that can result in better risk management up front and reduce potential cost from fraudulent activities or noncompliance in the long run. Emerging markets are showing awareness of cybercrime, but they have not committed as many resources to combating it as their counterparts in developed markets. Lack of funding appears to be a major constraint, but this also leaves companies at increased risk of an attack. The final picture of FDA in 2016 is of a technology that is slowly gaining traction and “ We need to provide better technical beginning to yield returns on investment. But companies have not yet thrown their full training to our people and improve the weight behind the paradigm, choosing instead to invest piecemeal and reap only some quality of information in our corporate of the benefits. In order to leverage the full power of FDA, firms need to commit, invest systems and applications.” 5and implement the strategy in its entirety. In doing so, they will join those that are already Risk Executive, Life Sciences, Brazil riding the crest of the analytics revolution. 37% of the respondents spend more than half of their FDA investment on reactive matters. Global Forensic Data Analytics Survey 2016 | 33
 Shifting into High Gear: Mitigating Risks and Demonstrating Returns Page 32 Page 34
Shifting into High Gear: Mitigating Risks and Demonstrating Returns Page 32 Page 34